That viscous film which envelops the Earth
Life on Earth barely extends over its surface. But organisms have been able to transform its climate for millions of years. Now, humans seem to reach it again in a record time.
![]()
To Joan.
There is a famous painting by the romantic Caspar David Friedrich in which a man faces a landscape of mountains enveloped in a hazy atmosphere. Some trees hardly stand out the profile of the mountains, evoking the green tapestry that covers the rocks. The picture illustrates well the dimension in which gea, biota and atmosphere interact. Mountain landscapes allow us to visualize the contrast between the huge size of the Earth, and the tiny biota, an amalgam of living beings that scarcely occupy the outer layer of the planet.

Experts say that life originated into the oceans when the Earth was barely 500 million years old, out of the 4.5 billion that it currently has. Since then life has not disappeared at any time. It has been expanding and diversifying with more or less success by all the earth holes. It can be found even in the last site of the oceans that fill the hollows of the lithosphere. It has even managed to penetrate the solid mass of ice and rocks to depths of several kilometers. Basically it has been achieved thanks to its extraordinarily dynamic chemical behavior, its metabolism. This allows life to reproduce in discrete units while it thermodynamically optimizes a convoluted network of chemical reactions. Life persists as it continues changing, remembering the Lampedusian principle.
But if we adopt the planet’s point of view, with its immense solid mass that reaches a diameter of 12,742 km, in reality life has barely scratched its surface. Life has speed Earth’s solid crust to chemically transform and erode. He has also changed the chemistry of his outer gas and liquid layers, but nothing more. These changes barely affect less than one millionth of the Earth's mass and one-thousandth of its diameter. But it has not affected at all its internal thermonuclear reactions nor the great movements of its surface masses, the continental plates, controlled by large convective cells of materials that appear solid, but which are somewhat fluid under high pressures and temperatures. Neither has life intervened at all in how the Earth moves through space, which is what actually determines the radiation that arrives to its surface and the impacts that it receives from other celestial bodies. We might think that for Earth, life is nothing more than a viscous film that has come to the surface and can not be cleaned. Even as the Earth changes while aging, tanned by the sun and losing the strength of its internal thermonuclear reactions, life quickly adapts to those geological changes, which are necessarily slower.
Now, we can look at those parts of the Earth that have been altered by that sticky live film. Probably his greatest success has been the transformation of the atmosphere, thanks to the oxygen released in photosynthesis. In fact, the main molecule involved, ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO) is considered to be the most abundant enzyme on Earth.

This transformation became really effective some 600 million years ago, when the atmospheric oxygen concentration reached 20% of the atmospheric gas mixture - a value similar to the present one, although much lower than the 35% that oxygen seems to have reached 300 Millions of years, at the end of the Carboniferous. Let us think that in its beginnings, the atmosphere practically lacked oxygen. Then life has oxygenated it. But it has been a slow process since life arose at a relatively early age on Earth - it is curious to think that the oldest crystal known is contemporary with those early living beings.
Oxygen resulting from photosynthesis began to be released into the atmosphere some 500 million years later, but did not attained fullness until 3,000 million years later, when living functional units —the organisms— reached a certain size. They had become more efficient and were able to assault the terrestrial environment, in direct contact with oxygen and with atmospheric CO2. This molecule is the great supplier of carbon, the fundamental piece of the "lego" that constitute the molecular architecture of life. The CO2 had been a rather pleasant and abundant tenant of the atmosphere since its inception. However, its role as a blocker of radiation escaping from the Earth had already been supplemented by methane, another molecule with carbon, surplus of biological activity prior to the great oxygenation. Life began to massively use CO2 and imprisoned it in sediments, sequestered on rocks for millions of years (an archetype, revisited in the Marvel pantheon). Now we humans are releasing him from the lithological prison where our biological ancestors had confined him. But we do it compulsively, too fast for our own interests.
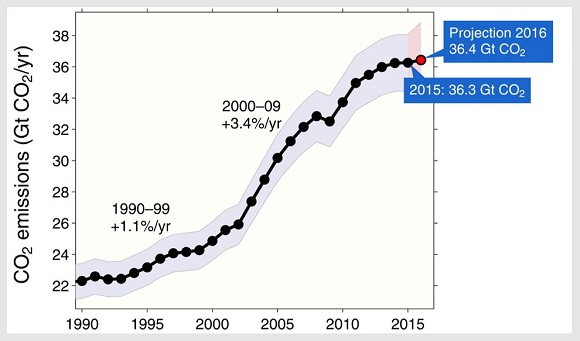
The increasing of CO2 in atmosphere has been extensively documented since the middle of the 20th century (see Global Carbon Project reports). We are at levels of CO2 concentration of about 400ppm (parts per million). Is that a lot? The answer is absolutely affirmative. It has been verified that the concentration of CO2 follows tightly the alternation of temperatures observed during the glaciations. In cold periods the values are about 200ppm while in the hotter periods between glaciations rise to about 280ppm. The ultimate reason for the alternation of glacial periods is the greater or lesser solar radiation that comes to Earth according to the regular changes that affect its orbit and its rotation —as Milankovitch proposed in the 1920s and statistically corroborated Hays, Imbrie and Shackleton in 1976. Then, CO2 contributes to retaining on Earth that radiation in a complex set of interactions with the photosynthesis biota, with the chemical processes that occur in the oceans and with the climate itself. Therefore, we are well above the usual values even in periods between glaciations.
Some recent studies have calculated that with the atmospheric CO2 that we already have accumulated we will be able to retain enough radiation to save at least the two glaciations that we would expect during the next two periods of low insolation, in a hundred thousand years. It might seem like something positive. Unfortunately, none of us will enjoy it. But before we will have to face tremendous problems to cool the daily life of the human population, to assure its water supply and to obstruct the rise of sea level, among other jokes. Curiously, these studies suggest that human activity would have already greatly increased the concentration of atmospheric CO2 before the industrial revolution, enough to save us from the next glaciation. How is it possible?
In fact, humans contribute to increasing atmospheric CO2 not only by burning coal, oil and natural gas but also by preventing vegetation from absorbing CO2 through photosynthesis. Logging forests and transforming them into crops, rangelands or wasteland has diminished the photosynthetic capacity of the biosphere. It has also promoted the return of carbon accumulated in the vegetation to the atmosphere. Today these processes represent 9% of the CO2 that should not be in the atmosphere but that it is there. This reduction of photosynthetic cover began significantly a few millennia ago and led to around 280 ppm of atmospheric CO2 before the industrial era. CO2 emissions accelerated during the second half of the 20th century thanks to the technological capacity to transform the landscape. With a single action —the destruction of forests— in addition to destroying biodiversity, we have been capable of altering the Earth's climate. The good news is that we can reverse this trend: in the temperate regions the forests recover, while in the tropics their destruction is no longer accelerated.
We have learned many of these things only recently. At the end of the 19th century, Swedish physicist Svante August Arrhenius has already described the greenhouse effect of gases such as CO2. Nevertheless, the global dimension of the phenomenon became clearly evident with the famous annual CO2 measurements made at the Mauna Loa Island Hawaiian Island Observatory, far from any significant source of emissions, starting in 1956.
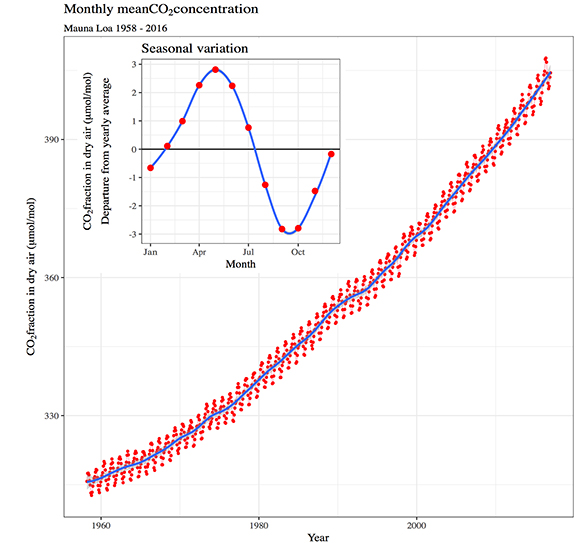
Since then, one of the most remarkable episodes in the history of science has developed. Scientists made their calculations —at first, in a rather simple way, today, in a extraordinarily complex and coordinated way— and reached a prediction that breaks schemas: Earth's global climate will change and warm as a result of human action. Until then the paradigm of the climate was based exclusively on the forces of nature and doubts still existed on the causes of the glaciations. Studies were largely devoted to interpreting regional climate patterns. In fact, the scientific community still has a small percentage —as much as 5%— of skeptics about the role of human activity in climate change. At that time, there were many more.
There was no evidence of warming, only predictions of a temperature rise in the medium term that were based on laboratory experiments and calculations that extrapolated them to the whole Earth. The prediction has been fulfilled, as shown by the instrumental data. It was not a prophecy, but a prediction based on knowledge. The scientists organized and explained their conclusions. We can imagine the resistances, which of course are not over yet. Although the results to reduce the CO2 emissions are clearly unsatisfactory, thanks to these predictions we have gained precious time. Without this knowledge we would be observing raising temperatures year after year and we intuitively or magically would attribute it to any cause, without being able to reverse the phenomenon. It was a milestone, a scientific prediction —in which a multitude of scholars participated— about a reality that affects the whole planet, or rather its outermost, thin layer.
To affirm that life is intimately linked with the Earth will not surprise anyone, it is a perception rooted in infinity of cultures. Observing the transformation of the landscape caused by humans is within the reach of all. But discovering that living beings have changed the physical properties of the Earth on a global scale, and that this transformation is almost as old as the planet itself is not at all obvious. We know this from scientific knowledge. Some geologists estimate that two-thirds of the 4,500 known minerals are an indirect result of biological activity as they were formed thanks to oxygen released by living beings. A recent study says that 208 new minerals have appeared since the origin of humans. With his idea of Gaia, Lovelock described well the intimate communion between the planet and the living beings it houses. But we must be a bit modest. The planet Earth follows its cosmic journey regardless the green and sticky film that envelops it. Life will hardly be taken away from Earth despite humans. Stephen Hawking states that the future of mankind is on other planets. Perhaps we would have to think seriously about, if we see that the changes we have introduced to Earth's climate system are beyond our control. Visiting and knowing other worlds can be quite a challenge, but it does not seem to be less than continuing to make the Earth habitable. And we know that humans like challenges, right?







