Humans milk the periodic table turning a blind eye to its risks
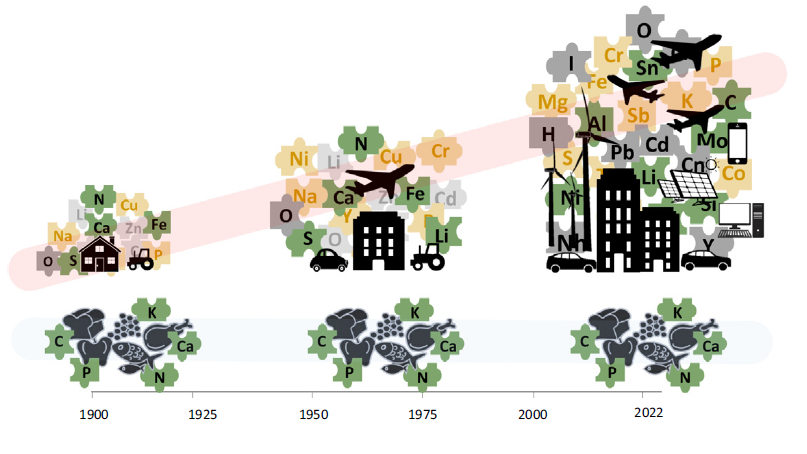
For millions of years, nature has basically been getting by with just a few elements from the periodic table. Carbon, calcium, oxygen, hydrogen, nitrogen, phosphorus, silicon, sulphur, magnesium and potassium are the building blocks of almost all life on our planet (tree trunks, leaves, hairs, teeth, etc.). However, to build the world of humans — cities, healthcare products, railways, aeroplanes and their engines, computers, smartphones — we need many more chemical elements. A recent article, published in the renowned journal Trends in Ecology and Evolution and written by researchers from CREAF, the Autonomous University of Barcelona (UAB) and the Spanish National Research Council (CSIC), warns that the range of chemical elements humans need (something scientifically known as the human elementome) is increasingly diverging from that which nature requires (the biological elementome).
“ Sustaining the human elementome will be more and more complicated and risky; it will need to be done in terms of environmental justice and, of course, with more rational use of Earth’s limited resources,”
JAUME TERRADAS, founder of CREAF, honorary professor at the UAB and one of the article’s three authors.
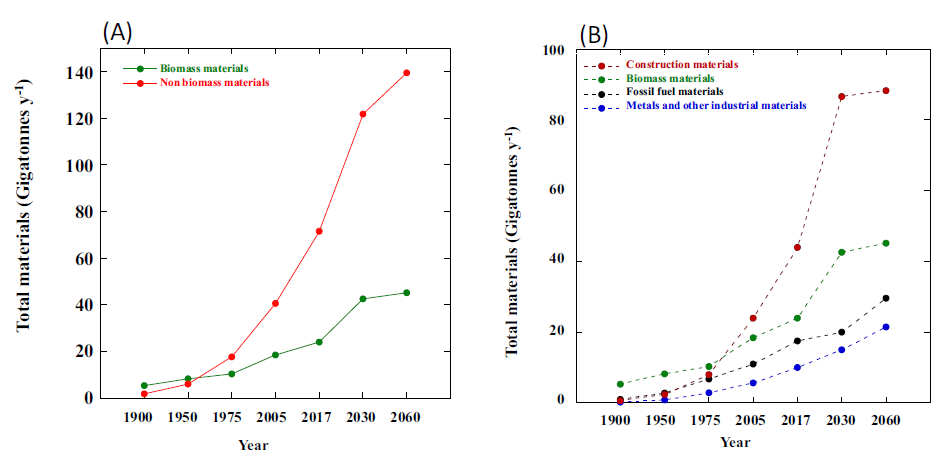
In 1900, approximately 80% of the elements humans used came from biomass (wood, plants, food, etc.). That figure had fallen to 32% by 2005, and is expected to stand at around just 20% in 2050. We are heading for a situation in which 80% of the elements we use are from non-biological sources. Non-biological elements are scarce or practically absent in living organisms, and rare in general; in many cases, their main reserves are located in just a handful of countries. They have to be obtained from geological sources, which entails extraction, trade between countries, and the development of efficient recycling technologies, while their scarcity and location create potential for social, economic, geopolitical and environmental conflicts. Thus, what might initially appear to be a purely scientific issue actually has much more far-reaching repercussions.

Humanity, firmly bound to its expansive use of the periodic table
The study looks back at the history of humankind in relation to its use of the periodic table’s elements. According to the article, the human and biological elementomes started to diverge in the decade of the 1900s, a result of continuous growth in the use of non-biomass materials (fossil fuels, metallic/industrial materials, and building materials).
“Humans have gone from using common materials, such as clay, stone and lime, the elements of which are constantly recycled in the ground, in nature and in the atmosphere, to using lots of other elements, notably including those known as rare-earth elements”JORDI SARDANS, CREAF and CSIC researcher and , co-author of the study

“Mineral element consumption/extraction is rising at a rate of around 3% a year, and that will continue up to 2050. In that scenario, it is possible that we will have used up all our reserves of some of those elements — gold and antimony — by 2050, and of others — molybdenum and zinc — within a hundred years.”
JOSEP PEÑUELAS, CREAF and CSIC researcher and co-author of the study

Previous
Next
In 1900, 79% of all the materials humans used annually were biomass materials, compared to 32% in 2005 and the figure of 22% currently predicted for 2050. Elements used in construction, transport, industry and, more recently, new technologies, such as computation and photovoltaic devices and mobile phones, were added to the human elementome over the course of the 20th century. They include silicon, nickel, copper, chromium and gold, as well as others that are less common, such as samarium, ytterbium, yttrium and neodymium. In the last two decades, there has been an increase in the use of such scarce elements owing to the implementation and expansion of new technologies and clean energy sources.
Environmental, economic, social and geopolitical risks
The extraction of Earth’s chemical elements could be a limiting factor and lead to crises at every level
The article leaves no room for doubt: the extraction of Earth’s chemical elements could be a limiting factor and lead to crises at every level. Using more of the periodic table’s elements involves the extraction of more minerals, rising energy consumption and the associated CO2 emissions. Furthermore, the growing scarcity of the elements in question is a threat to their availability, especially where poorer countries are concerned, and makes maintaining production difficult even for wealthy countries, thus affecting economic development.

Against that backdrop, there are also important and problematic geopolitical considerations. The natural reserves of some elements, including the rare-earth elements, are located in a limited number of countries (China, Vietnam, Brazil, the USA, Russia and the Democratic Republic of the Congo); China actually controls over 90% of the global supply and close to 40% of reserves. Their availability is therefore subject to fluctuations in supply and prices caused by opposed geopolitical interests, with the consequent danger of conflicts.
Out with programmed obsolescence, in with recycling and recovery
The authors stress the need to put an end to programmed obsolescence (the policy of planning or designing a product to have an artificially limited useful life), as well as to develop new technologies that contribute to more profitable use of scarce elements and allow for their widespread, efficient recycling and reuse. At present, there are few, if any, alternatives to many such elements, and their recycling rates are low because they are used in small amounts in combination with other materials in a broad range of products. Current recovery techniques have poor efficiency levels and entail a high risk of pollution owing to the toxicity of rare-earth elements.

The article mentions different technologies that could be used for the recovery of scarce elements. One is bioleaching, the extraction of metals from their ores through the use of living organisms, such as bacteria, which can accumulate rare-earth elements if they come into contact with industrial waste. To avoid pollution, meanwhile, scientists are studying biosorption, a physiochemical process that occurs naturally in certain organisms and enables them to filter pollutants, such as heavy metals in waste water. Other possibilities include cryomilling, in which recovery takes place through electrochemical deposition; the use of different carbon-based nanomaterials as means of sorption to preconcentrate rare elements from waste water from dissolved waste; hydrometallurgy for the substantial recovery of rare-earth elements and heavy metals from apatite and different scraps; and pyrometallurgy or the extraction of supercritical fluids with CO2. In any case, developing new ways of producing and recycling such elements on a large scale is essential.
Article:
Penuelas, J., Sardans, J., & Terradas, J. (2022). Increasing divergence between human and biological elementomes. Trends in Ecology & Evolution.








